Download Factsheet
Choose your language:

Successfully managing all contracts that are required to perform a clinical study
About us
- An international team of dedicated lawyers
- Industry experience
- Country-specific knowledge
- Flexible contracting resources
- Timelines, quality and risk management
- ICH-GCP trained staff
Our services
- Site contracting & budget negotiations
- Service agreements with study vendors
- Review of other contracts & study documents
- Contracting strategy and planning
- Contracting rescue management
- Contract dispute resolution support
- Data privacy solutions for your clinical trials (GDPR)
Why Salvius?
- Improve contracting timelines, whilst maintaining quality and reduce risk
- Protection of your rights in contracts for clinical trials
- Building successful relationships with stakeholders
- Customized support, using a collaboration model that fits your specific needs
- Proper oversight and control over your contracting process
Global coverage
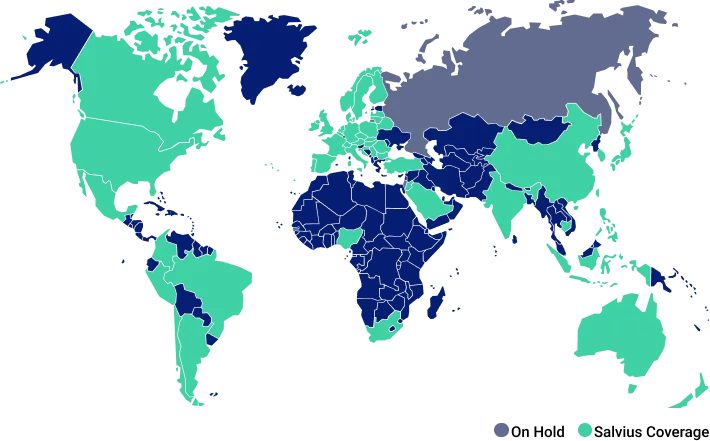
By the numbers
5000+ agreements
50+ countries
250+ studies
For all study types
Drug studies
Medical device studies
Medtech studies
Medical food studies
Registry studies
Every phase
In every product development phase